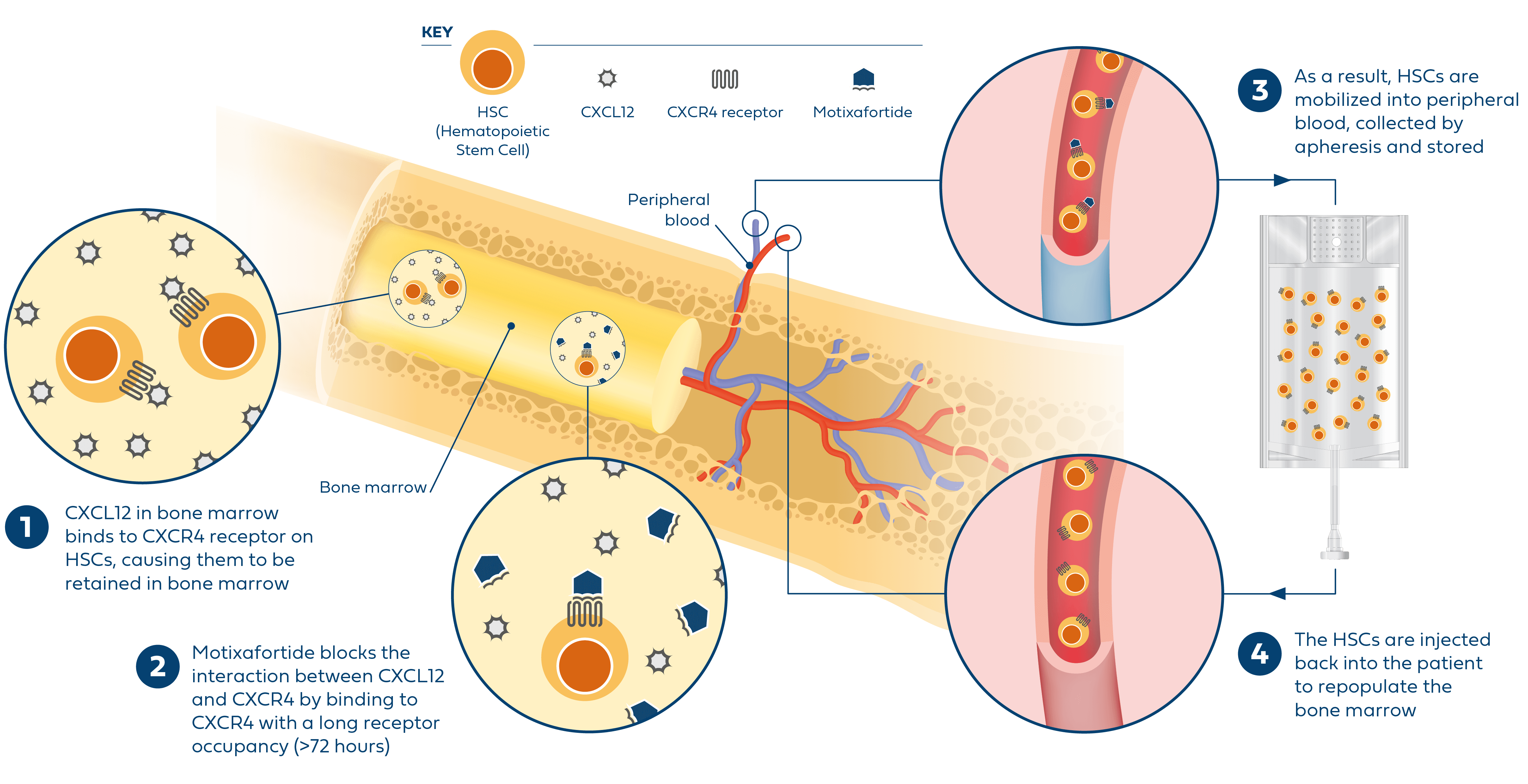
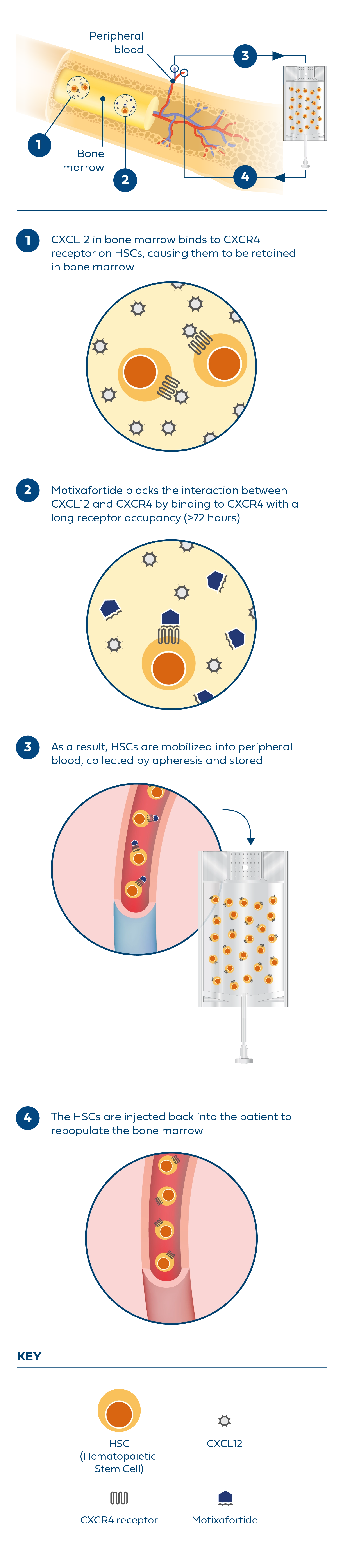
References: 1. Dhakal B, Shah N, Kansagra A, et al. ASTCT clinical practice recommendations for transplantation and cellular therapies in multiple myeloma. Transplant Cell Ther. 2022;28(6):284-293. 2. Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308. 3. Swan D, Hayden PJ, Eikema DJ, et al. Trends in autologous stem cell transplantation for newly diagnosed multiple myeloma: changing demographics and outcomes in European Society for Blood and Marrow Transplantation centres from 1995 to 2019. Br J Haematol. 2022;197(1):82-96. 4. Morris CL, Siegel E, Barlogie B, et al. Mobilization of CD34+ cells in elderly patients (>/= 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol. 2003;120(3):413-423. 5. Bolon YT, Atshan R, Allbee-Johnson M, Estrada-Merly N, Lee SJ. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides, 2022. Accessed August 8, 2023. https://cibmtr.org/Files/Summary-Slides--Reports/The-US-Summary-Slides-2022-v3---web-version.pptx. 6. Hulin C, Offner F, Moreau P, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica. 2021;106(8):2257-2260. 7. Chhabra S, Callander N, Watts NL, et al. Stem cell mobilization yields with daratumumab- and lenalidomide-containing quadruplet induction therapy in newly diagnosed multiple myeloma: findings from the MASTER and GRIFFIN trials. Transplant Cell Ther. 2023;29(3):174.e1-174.e10. 8. DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-5726. 9. Edmisson J, et al. Poster presented at: 64th American Society of Hematology Annual Meeting and Exposition; December 10-13, 2022; New Orleans, LA. 10. Shaughnessy P, Chao N, Shapiro J, et al. Pharmacoeconomics of hematopoietic stem cell mobilization: an overview of current evidence and gaps in the literature. Biol Blood Marrow Transplant. 2013;19(9):1301-1309. 11. Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308. 12. APHEXDA. Prescribing information. BioLineRx Ltd; 2023.